Abstract
Background: Approximately 50% of patients receiving CAR-T cells will relapse, with half of these relapses due to CD19neg antigenic escape. To address this, we have explored an alternative pathway for targeting antigen-negative leukemic cells through activated endogenous CARneg bystander T cells, which could provide additional leukemic control through CAR-independent mechanisms. Here we leverage data from a NHP CAR-T cell model, and from clinical studies, to identify mechanisms of CARneg T cell bystander activation and their potential antileukemic role.
Methods: NHP: Anti-CD20 CAR-T cells were transferred into lymphodepleted NHP as previously described (PMID: 29563103). Expansion of these cells resulted in B cell aplasia, and induced clinical Cytokine Release Syndrome (CRS). The transferred CAR-T cells persisted for ~4 weeks, at which point loss of CAR-Ts was followed by B cell recovery. Flow cytometry, scRNA- and scTCR Seq were performed on 5 NHP CAR-T cell recipients. Clinical Samples: Flow cytometry, scRNA- and scTCR-Seq were also performed on T cells from 6 pediatric B-ALL patients receiving Tisangenlecleucel. ScRNA-Seq and scTCR-Seq libraries were aligned with cellranger, preprocessed and analyzed with the Python packages scanpy and scVI.
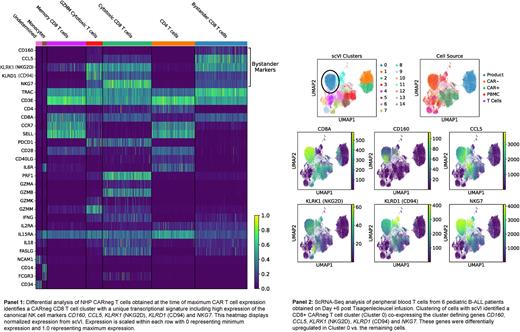
Results: NHP recipients of CD20-CAR T cells demonstrated CAR-T cell maximum expansion on Days 7-11 post-infusion. Coincident with CAR-T cell expansion, we also observed a 5-fold expansion of activated, CD8+ CARneg T cells. To rigorously profile the expanded CARneg T cells, we performed scRNASeq on sorted CARneg T cells from the infused product, at the time of maximum CAR-T proliferation (Day 11), and during the CAR-T contraction phase (Day 20). Longitudinal clone tracking and associated gene expression analysis demonstrated that the CD8 CARneg T cell clones formed a unique cluster that emerged in parallel with expansion of CARpos T cells. This CD8 CARneg population made up <1% of T cells in the blood prior to CAR-T cell infusion or in the infused CAR-T cell product, suggesting evolution and expansion of these cells in the post-CAR-T cell infusion/CRS milieu. These 'bystander' CD8 CARneg T cells demonstrated high expression of the cytokine receptors for IL15 and IL2, and of the cytokines IL-18, IL2 and IFNg, along with expression of canonical cytotoxicity molecules, including Perforin and Granzyme M. In addition, the CARneg CD8 T cells expressed high levels of KLRK1 (NKG2D) and FASL, receptors that have been associated with direct tumor lysis. T cell repertoire analysis demonstrated high TCR diversity, suggesting that these cells were bone fide T cells, and not NK-T or MAIT cells. To better understand this population, we subsetted all clusters enriched for CARneg CD8 T cells at the time of maximum CAR-T cell proliferation and reclustered this data, which enabled the further identification of these CARneg CD8 T cells to express multiple NK markers, including KLRK1 (NGG2D), KLRD1 (CD94), CD160, CCL5, NKG7 (Panel 1), a transcriptional signature that is similar to CD8 T cells recently found to emerge as cytotoxic bystander cells in viral infections (PMID: 31827070), and in the inflammatory tumor microenvironment (PMID: 33468558), where they provide additional anti-viral/tumor control.
We next performed scRNA-Seq on samples from 6 patients receiving Tisagenlecleucel CAR-T cells. At the time of maximal CAR-T cell expansion, we identified a CD8 CARneg T cell population in all patients, which mirrored the transcriptional signature originally observed in NHP (Panel 2). To determine whether cytokines released by CRS could induce the transformation of human CD8 CARneg T cells into these highly activated cells, we stimulated primary human T cells with an array of CRS-associated cytokines, and found that the gamma cytokines IL-2 and IL-15 induced a CD8+ T cell population that upregulated a similar group of phenotypic markers (CD160, NKG2D and CCL5) observed in the bystander cells obtained from CAR-T cell patients.
Conclusions: These data demonstrate for the first time that a CAR-T cell induced bystander effect is predominantly elicited in CD8 CARneg T cells, in both NHP and patients. These CD8 CARneg T cells express several canonical activation and NK markers in response to CRS-associated cytokines, and also express both NKG2D and FASL, suggesting that they could contribute to anti-leukemic effects through CAR-independent mechanisms.
Disclosures
Gerdemann:AlloVir: Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Shalek:Wellcome Leap: Research Funding; Novo Nordisk: Research Funding; Janssen: Research Funding; Leo Pharma: Research Funding; Novartis: Research Funding; Dahlia Biosciences: Consultancy; IntrECate biotherapeutics: Consultancy; Senda Biosciences: Consultancy; Relation Therapeutics: Consultancy; Empress Therapeutics: Consultancy; FL82: Consultancy; Ochre Bio: Consultancy; Third Rock Ventures: Consultancy; Hovione: Consultancy; Repertoire Immune Medicines: Consultancy; Cellarity: Consultancy; Honeycomb Biotechnologies: Consultancy; Merck: Consultancy, Research Funding. Kean:Mammoth Biosciences: Current equity holder in private company; Vertex: Consultancy; EMD-Serono: Research Funding; Bluebird Bio: Research Funding; HiFiBio: Consultancy; Bristol Myers Squibb: Research Funding; Magenta: Research Funding; Kite/Gilead: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal